Regulatory Artwork & Packaging Design
Transform complex compliance requirements into market-ready visual solutions that enhance your brand while ensuring global regulatory approval. Where technical precision meets creative excellence.

Transform complex compliance requirements into market-ready visual solutions that enhance your brand while ensuring global regulatory approval. Where technical precision meets creative excellence.

From initial concept to final submission, we deliver artwork and packaging solutions that meet the most stringent regulatory requirements while preserving your brand identity.
Complete artwork packages prepared to regulatory authority specifications with all required documentation and technical files.
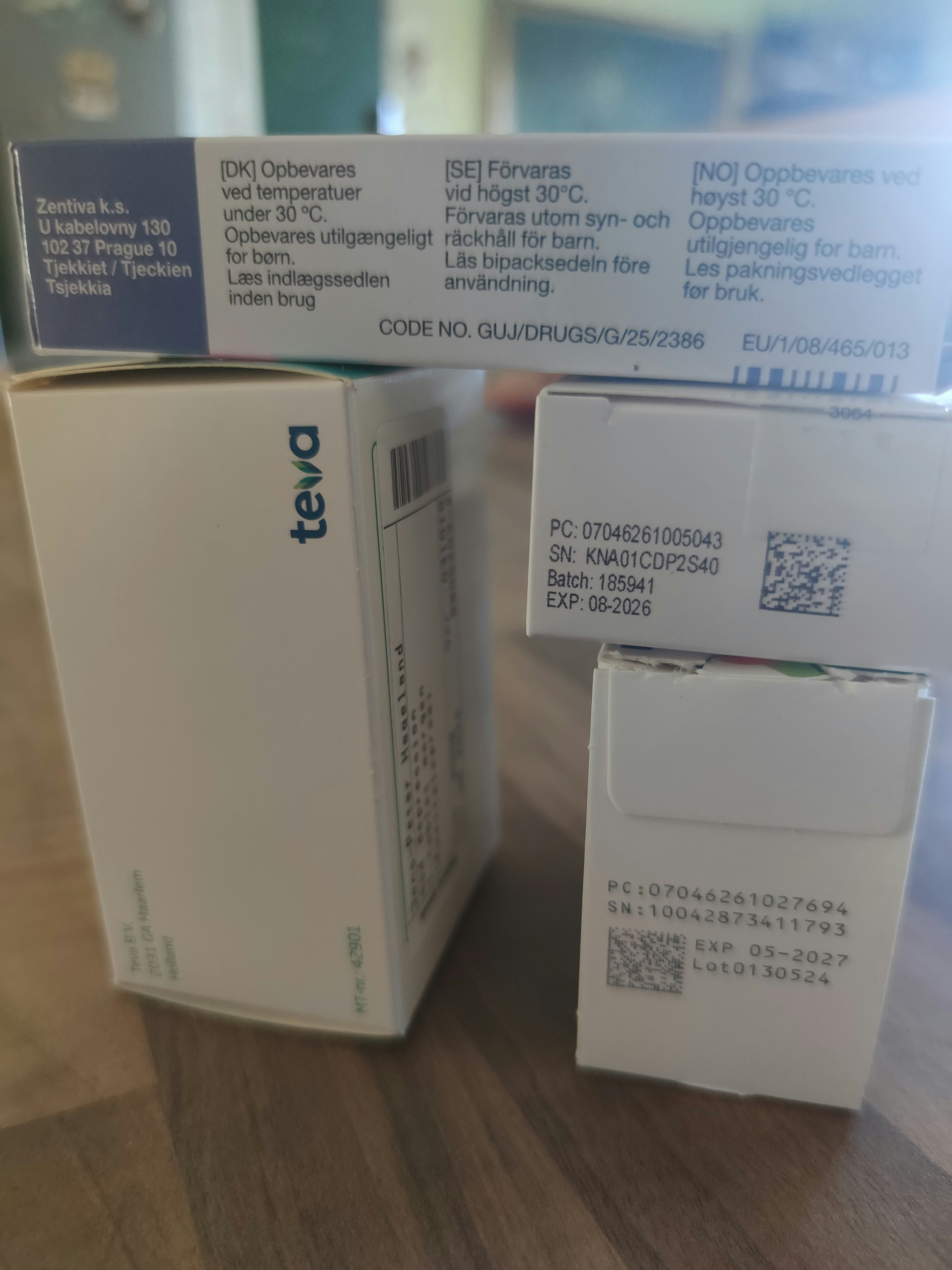
Expert handling of complex multilingual requirements with culturally appropriate typography and layout optimization.
Photorealistic 3D renderings and mockups for stakeholder approval and marketing material preparation.
Comprehensive regulatory review and verification against jurisdiction-specific requirements before submission.
Streamlined revision processes for regulatory feedback incorporation with version control and change tracking.
Seamless integration of existing brand guidelines with regulatory requirements to maintain brand consistency.
See how we transform non-compliant designs into submission-ready artwork while enhancing brand appeal and market readiness.
A systematic approach to regulatory design excellence
Comprehensive review of jurisdiction-specific requirements and existing brand guidelines.
Seamless integration of compliance requirements with brand identity preservation.
Multi-point verification against regulatory standards and submission requirements.
Complete artwork package with technical specifications and regulatory documentation.
Our systematic approach ensures every regulatory requirement is met across all major jurisdictions.
Download our comprehensive 50-point regulatory artwork checklist covering all major jurisdictions.
Download Free ChecklistTell us about your regulatory artwork needs and receive a detailed quote within 24 hours.